
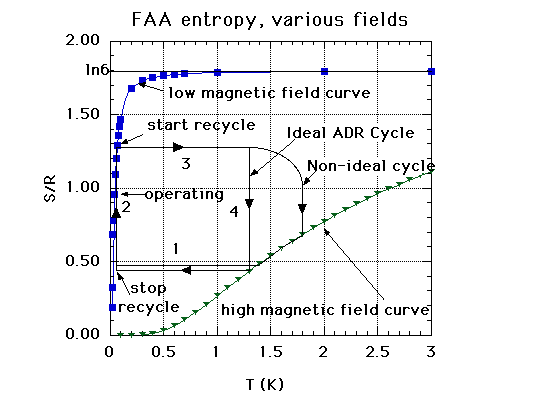
Temperature Entropy Graph
This figure is a graph of entropy vs. temperature for Ferric Ammonium Alum (FAA). The horizontal axis is temperature in degrees Kelvin, from 0 to 3. The vertical axis is Entropy, in a unitless form created by dividing the specific entropy by the gas constant R. This dimensionless entropy runs from 0 to 2.
The graph shows:
- entropy of FAA at low magnetic field
- entropy of FAA at high magnetic field
- path of of an ADR salt pill over this diagram as the ADR runs through its cycle
Entropy at Low Magnetic Field
When the temperature is zero, the entropy of the FAA is zero. (Any perfect crystal at absolute zero has zero entropy.) Entropy rises quickly with temperature. By about 0.5 Kelvin, the entropy has leveled off at the natural log of 6 (about 1.8), which value it keeps to the top of the graph.
Entropy at High Magnetic Field
At zero Kelvin, entropy in high magnetic field is also zero. As the temperature rises, the high field entropy rises more slowly than the low field entropy. (The applied magnetic field imposes order on the molecular magnetic moments and thus limits their entropy.) By 3 Kelvin, the high field entropy has climbed to about 1.1, or about two thirds of the low field entropy at that temperature.
Ideal ADR Cycle
The changes in temperature and entropy that the salt pill undergoes during the cycle can be plotted as a path on the temperature-entropy graph. The cycle has 4 sections:
- The horizontal line marked "1" on the graph shows what happens in the part of the cycle where the high magnetic field is rapidly dropped to a low value and the salt pill responds by cooling down rapidly. This part of the path starts at the high magnetic field curve at a temperature of 1.3 Kelvin and an entropy of about 0.4. From there, the path goes left, that is, to lower temperature. It stops just before it reaches the low magnetic field curve, at a temperature of about 0.1 Kelvin.
- The vertical line, marked "2" and "operating", shows the part of the cycle where the ADR is actively involved in cooling. When I say that this line segment is vertical, I mean that it is at constant temperature. (Remember that temperature is plotted on the horizontal axis.) This line starts where line 1 left off, that is, at a temperature of about 0.1 Kelvin and an entropy of about 0.4. It rises straight up till it intersects the curve that shows the low magnetic field entropy of the salt pill. During this part of the cycle, the salt pill is absorbing heat and thus increasing its entropy. As the entropy increases, it moves up the graph. (Remember that entropy is plotted on the vertical axis.) The operators hold the temperature constant by carefully and slowly decreasing the applied magnetic field to compensate for the added heat. The point where this line intersects the low magnetic field curve represents the time when the operators have decreased the field produced by their magnet to zero. No field is left, other than the weak self-field of the salt and any stray fields. Since the operators can no longer hold the temperature constant, they recycle, that is, they warm up the salt pill to dump the stored heat.
- The horizontal line, marked "3" on the graph, shows the rapid warm-up part of the cycle. It starts at the end of the previous line, that is at a temperature of 0.1 Kelvin and an entropy of about 1.25, and moves horizontally to the right, that is, to higher temperatures. It ends at a temperature of about 1.3. This line segment represents what happens in the salt pill as operators increase the magnetic field, warming the salt pill so that it can dump the stored heat to the warm end heat sink.
- The vertical line, marked "4" on the graph, shows the decrease in entropy at constant temperature as the salt pill dumps its heat to the heat sink. In an ideal ADR, the temperature would indeed be held constant during the time that the heat switch allowed heat to flow from the salt pill to the heat sink. This line moves down the graph, ending where line 1 started, that is at a temperature of 1.3 Kelvin and an entropy of 0.4. At the end of this segment, the heat switch is turned off, so that, after the salt pill cools down, heat cannot flow back into the salt pill from the heat sink.
Non-Ideal Cycle
The non-ideal cycle is the same as the ideal cycle except in part 4 and the end of part 3, when it is dumping the heat to the heat sink. The non-ideal cycle is not shown by vertical line, that is, the process does not happen at constant temperature. At the end of segment 3, the warm up, the non-ideal path goes to a higher temperature. By raising the salt pill temperature to a higher temperature than the heat sink, the operators can dump the heat more quickly (though the process is not quite as efficient.) They turn on the heat switch before the salt pill reaches its highest temperature, though, and heat starts flowing into heat sink before they stop ramping the temperature up. Because the heat starts flowing before the salt pill finishes warming, the non-ideal path curves from segment 3 to segment 4. In the ideal cycle, there is no curve, only a sharp corner, since the ideal heat switch can turn on instantaneously when the ideal salt pill reaches the temperature of the heat sink.
The non-ideal cycle also differs from the ideal at the end of the heat dumping part of the cycle. The non-ideal cycle intersects the high magnetic field entropy curve, and follows it down in entropy and temperature to the point where it intersects line segment 1. This represents the fact that the non-ideal cycle continues to dump heat into the heat sink as the magnetic field begins to ramp down.
Return ADR Cycle Page
